Our Capabilities
Neurodevelopment Research at MRN
A neurodevelopmental disorder is one that impairs a child’s ability to develop along a typical trajectory. While the symptoms and causes of these disorders can vary widely, targeted interventions generally lead to better outcomes for these children. Since the brain is most malleable in children less than five years of age, the earlier interventions begin, the more likely these children will follow a closer to normal developmental path. This will hopefully lead to improved quality of life for these children and their families by increasing the child’s independence as well as reducing long-term medical costs. One of the barriers to early identification is that many disorders are diagnosed behaviorally, which is limiting due to the natural variability in behavior among healthy children. Behavioral data often fail to indicate which interventions will be most appropriate for a given child, so understanding the atypical brain responses may help to develop and guide the use of a specific treatment.
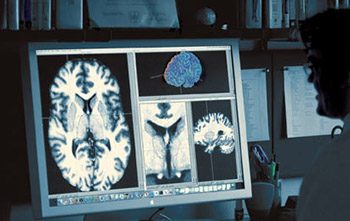
Using noninvasive imaging, MRN investigators are studying brain development from birth through childhood with the goal of using brain structure and function to identify markers of disorders for the purpose of guiding therapies. Beginning with specific disorders, progress has been made on several fronts, including mapping epileptic activity in young children and identifying structural and functional differences in children born prematurely. Using the world’s first pediatric MEG system (the babySQUID®), Dr. Julia Stephen and her team have identified a measure of atypical brain connectivity in children as young as 20 months who have been diagnosed with an autism spectrum disorder (ASD). Identification of specific markers in children already diagnosed with an ASD may help identify markers of atypical brain development in young children who may be at risk for developing this disorder. Dr. Stephen is also focusing on identifying markers in children aged three to five years with fetal alcohol spectrum disorders (FASD). Due to the stigma associated with drinking alcohol during pregnancy, many children are not diagnosed with FASD until as late as five years of age. Like ASD, early intervention is key to improving the quality of life of children with an FASD, who are known to be at higher risk for developing other mental disorders. Please visit Dr. Stephen's Child Study website.
With funding from the Delle Foundation, Drs. Arvind Caprihan and John Phillips are collaborating on a large scale project to investigate how MRI can be used to better understand normal brain development in children from birth to five years of age. In addition to performing a normative longitudinal study on children in the first year of life, Drs. Caprihan and Phillips are collaborating with University of New Mexico researchers on a new and important NIH study to better understand the structural and functional changes associated with providing erythropoietin to premature children in neonatal intensive care.
The neurodevelopment team also includes senior investigator Dr. Jeffrey Lewine. His autism research focuses on identifying markers that will help identify optimal intervention techniques for individual children. He is currently conducting a tinnitus study examining the safety and efficacy of a new procedure for reducing tinnitus using Tinnitus Re-Organization Training (TROT). Through these combined efforts, MRN neurodevelopment researchers are working toward improving the identification of developmental disorders and optimizing interventions based on known anatomical and/or functional deficits.
Last year was particularly good for the growth of our addiction research area. Collaborative efforts among MRN and other scientists combine neuroimaging and genetic approaches to identify genes that influence the progression of addiction that could eventually guide treatment and prevention efforts. Dr. Kent Hutchison leads the addiction research team and recently received a five-year grant to examine the neurobiological effects of the medication varenicline on smoking cessation. Also, Dr. Sarah Feldstein Ewing received a five-year grant to study risky behaviors in adolescents along with two exploratory grants to develop new treatment approaches using fMRI. Dr. Vince Clark’s study of recovering cocaine and methamphetamine addicts has led to the discovery of possible differences in brain structure between those who relapse and those who do not. When comparing MRI scans of abstainers and relapsed users, abstainers showed greater activity in an area of the brain associated with emotion and attention. “One function this area of the brain handles is interpreting the world around you in terms of how it affects your health and safety,” Dr. Clark explains. These scans, when combined with psychological histories of recovering stimulant addicts, have so far been 90% accurate in predicting relapses–hopefully leading to new rehabilitation techniques.
Drs. Matthew Shane and Eric Claus are conducting an fMRI study to see how past drug use influences brain function in previously incarcerated individuals. Visit the study website by clicking here.
Going forward, MRN addiction researchers will be investigating new methods to predict, diagnose and treat addictions using combined genetic and neuroimaging analyses. Specifically, proposals have been submitted to study positive and negative reinforcement processes involved in pain, cocaine addiction and relapse, smoking, eating, and cannabis use.

As neuroimaging research continues to grow, dynamic neuroinformatics systems are necessary to store, retrieve, mine and share the mass amounts of data. COINS has been created to facilitate communication and cultivate a data community by improving the ability to share data as well as research tools. This tool suite enables rapid sharing of data among PIs, querying of data types and assessments, and real-time reporting, among other functionalities. It manages studies at the Mind Research Network, the Nathan Kline Institute, University of Colorado - Boulder and the Olin Neuropsychiatry Research Center. COINS is dynamic and develops as the neuroimaging field grows.
COINS Tools
MICIS
DICOM Receiver
Assessment Manager
Self Assessment
Tablet
Query Builder
Portals
CAS
My Security
Please visit coins.mrn.org for more information and a demo of the COINS database!
 Autism Research at MRN
Autism Research at MRNLed by Drs. Jeffrey David Lewine and Julia Stephen, autism research at MRN spans an age range from toddlers through adulthood, with studies exploring all levels of severity for the autism spectrum disorders. Supported by an RO1 grant from the National Institute of Child Health and Development, Dr. Lewine has been studying the relationships between auditory processing, epilepsy, and language skills in autism. This research is designed to better understand language dysfunction in autism for the purpose of developing better therapeutic strategies. These studies involve detailed diagnositc, cognitive, and imaging assessments, with feedback provided to the family. Dr. Stephen’s research, also previously funded by the National Institutes of Health, uses brain imaging and simple sensory paradigms to probe early indications of atypical brain connectivity in children with autism as young as 15 months of age. The long-term goal of this work is to find markers of atypical brain development that can be utilized in infants prior to diagnosis to allow for the earliest intervention possible.
In addition to research on the basic biology of autism, the MRN team is directly involved in the development and evaluation of new treatment strategies. Dr. Lewine has received an award from the NIH to develop a new music-based therapy – Auditory Processing Training – to ameliorate sound hypersensitivity and other auditory processing problems in autism. He is also working with a California company on the development of a special EEG cap to enable sleep studies at home, to look for sleep problems and possible night-time epileptic and seizure activity. Dr. Lewine is also a site Principal Investigator on a clinical trial looking at the ability of Memantine to improve core social skills, and he is actively researching other treatment strategies including Auditory Integration Training, Irlen Colored Glasses, Interactive Metronome, and EEG-based Neurofeedback.
MRN’s forensic program continues to expand in both size and scope as investigators try to shed light on the underlying causes of anti-social behaviors. Crime is estimated to cost society more than $2.3 trillion a year, and MRN researchers are hoping to discover some of the neurobiological correlates of the behaviors that cause this huge economic and societal burden.
MRN’s forensic research team now involves more than 40 investigators and staff. Through the use of a unique mobile MRI system, over 2000 brain scans have been collected on location at adult and adolescent correctional facilities, representing the largest set of neuroimaging data ever collected from forensic populations.
Dr. Kent Kiehl is the lead scientist of MRN’s forensic program and the Director of MRN’s mobile imaging core. “Our studies lead us to believe psychopathic behavior results from disruptions to the parts of the brain that regulate emotion, attention, decision making and other cognitive functions,” Dr. Kiehl said. When presented with a series of words intended to invoke an emotional response, psychopaths show deficits in the paralimbic regions when processing ‘emotional’ words but show greater activity in lateral frontal areas, which are considered classic language areas. “Putting it simply, they use emotional regions less, and tend to employ non-emotional areas to do the processing,” Kiehl explains. “Once we understand what causes abnormal behaviors, we can then work to more effectively diagnose and treat these disorders.” Dr. Kiehl is also a member of The MacArthur Foundation Law and Neuroscience Project, which is studying how neuroscience is changing the legal system.
Another member of the team, Dr. Carla Harenski, is expanding on Kiehl’s research by studying social cognition and moral judgment in psychopaths. “The psychopath’s emotional deficits interfere with the development of normal moral socialization, beginning in childhood. As a result, they are incapable of appreciating the consequences of committing harmful, immoral actions.” While the brain systems underlying moral judgment in non-antisocial populations have been established, how these systems function in psychopaths is still unknown. “With the mobile scanner, we can now explore these questions by studying incarcerated psychopaths.” In Dr. Harenski’s recent work, she has found that psychopaths, relative to non-psychopaths, show abnormal activity in paralimbic brain regions when they view pictures depicting moral violations (e.g. one person intentionally causing harm to another).
Harenski plans to extend her research by combining fMRI with functional genomic analyses, by including other forensic populations, such as female psychopaths and sex offenders, and by examining the effects of psychopathy and substance abuse on moral decision making. To fund this ambitious agenda, a center grant focused on the neurobiology and genomics of psychopathy and substance abuse is in preparation.
A neurodevelopmental disorder is one that impairs a child’s ability to develop along a typical trajectory. While the symptoms and causes of these disorders can vary widely, targeted interventions generally lead to better outcomes for these children. Since the brain is most malleable in children less than five years of age, the earlier interventions begin, the more likely these children will follow a closer to normal developmental path. This will hopefully lead to improved quality of life for these children and their families by increasing the child’s independence as well as reducing long-term medical costs. One of the barriers to early identification is that many disorders are diagnosed behaviorally, which is limiting due to the natural variability in behavior among healthy children. Behavioral data often fail to indicate which interventions will be most appropriate for a given child, so understanding the atypical brain responses may help to develop and guide the use of a specific treatment.
Using noninvasive imaging, MRN investigators are studying brain development from birth through childhood with the goal of using brain structure and function to identify markers of disorders for the purpose of guiding therapies. Beginning with specific disorders, progress has been made on several fronts, including mapping epileptic activity in young children, and identifying structural and functional differences in children born prematurely. Using the world’s first pediatric MEG system (the babySQUID®), Dr. Julia Stephen and her team have identified a measure of atypical brain connectivity in children as young as 20 months who have been diagnosed with an autism spectrum disorder (ASD). Identification of specific markers in children already diagnosed with an ASD may help identify markers of atypical brain development in young children who may be at risk for developing this disorder. Dr. Stephen is also focusing on identifying markers in children aged three to five years with fetal alcohol spectrum disorders (FASD). Due to the stigma associated with drinking alcohol during pregnancy, many children are not diagnosed with FASD until as late as five years of age. Like ASD, early intervention is key to improving the quality of life of children with an FASD, who are known to be at higher risk for developing other mental disorders. Please visit Dr. Stephen's Child Study website.
With funding from the Delle Foundation, Drs. Arvind Caprihan and John Phillips are collaborating on a large scale project to investigate how MRI can be used to better understand normal brain development in children from birth to five years of age. In addition to performing a normative longitudinal study on children in the first year of life, Drs. Caprihan and Phillips are collaborating with University of New Mexico researchers on a new and important NIH study to better understand the structural and functional changes associated with providing erythropoietin to premature children in neonatal intensive care.
The neurodevelopment team also includes senior investigator Dr. Jeffrey Lewine. His autism research focuses on identifying markers that will help identify optimal intervention techniques for individual children. He is currently conducting a tinnitus study examining the safety and efficacy of a new procedure for reducing tinnitus - Tinnitus Re-Organization Training (TROT). Through these combined efforts, MRN neurodevelopment researchers are working toward improving the identification of developmental disorders and optimizing interventions based on known anatomical and/or functional deficits.
With MRN’s highly advanced tools and techniques, we have been able to expand the research scope beyond typical neuroimaging. A few of the specialized research areas include, oral orthotics, brain stimulation (TDCS), learning, memory, aging and research ethics. MRN’s imaging services, capabilities and expertise allow our investigators the freedom to pursue groundbreaking research ideas.

As neuroimaging research continues to grow, dynamic neuroinformatics systems are necessary to store, retrieve, mine and share the mass amounts of data. COINS has been created to facilitate communication and cultivate a data community by improving the ability to share data as well as research tools. This tool suite enables rapid sharing of data among PIs, querying of data types and assessments, and real-time reporting, among other functionalities. It manages studies at the Mind Research Network, the Nathan Kline Institute, University of Colorado - Boulder and the Olin Neuropsychiatry Research Center. COINS is dynamic and develops as the neuroimaging field grows.
COINS Tools
MICIS
DICOM Receiver
Assessment Manager
Self Assessment
Tablet
Query Builder
Portals
CAS
My Security

 Psychosis Research at MRN
Psychosis Research at MRNSchizophrenia and other psychotic disorders remain a primary research focus for MRN researchers and collaborators. Great progress has already been made towards the goal of finding earlier and better methods to diagnose these diseases and bring more personalized treatment to the individuals suffering with these disorders.
For example, MRN researchers moved one step closer towards the development of a new diagnostic tool to accurately classify patients with schizophrenia and bipolar disorder, a historically difficult diagnosis. Using fMRI scans (which essentially gives a picture of changes in blood flow over time), Dr. Vince Calhoun and his team have been able to differentiate between the two disorders with better than 90% accuracy. Networks in the brain can be identified using fMRI, thus allowing researchers to see locations and patterns of brain activation while participants are doing a task as opposed to when they are at rest. Dr. Calhoun explains, “When looking at schizophrenia and bipolar disorder, we found two regions that showed profound differences in schizophrenia. Once we identified these networks, we were able to look at whether the changes in these areas could help us better differentiate schizophrenia from bipolar disorder, and also from healthy individuals.” This discovery may eventually provide clinicians with more objective methods to accurately differentiate and diagnose these disorders earlier in the disease progression, which could result in the application of more effective treatments and better long-term prognoses.
Our investigators have developed additional mechanisms for identifying linked changes in large-scale genetic and functional brain imaging data. Using these tools, the relationship between multiple genetic markers and brain activation patterns in schizophrenia were identified. Ongoing research indicates this relationship is different for schizophrenia patients than for healthy controls, which suggests a fundamental difference in the brain organization between these two groups.
Another major accomplishment was MRN researchers receiving an $11M COBRE center grant. The grant will support four projects designed to identify key neurobiological mechanisms related to schizophrenia. The hope is that the results of these studies will eventually be used to facilitate the early diagnosis of schizophrenia and the reliable prediction of treatment outcomes.
One of MRN’s initial projects was the MIND Clinical Imaging Consortium (MCIC), a multi-institutional study of first-episode and chronic schizophrenia patients. MCIC has created one of the world’s largest and most comprehensive collections of anatomical and functional MRI, MEG, and biogenetic data related to schizophrenia. MCIC data have been reported in numerous publications each year, and MRN researchers are expanding the consortium with new collaborations and innovative research methods.
Goals for the MRN schizophrenia research team include developing new methods for predicting cognitive function in schizophrenia, leveraging existing studies on young people facing psychosis into a new imaging and multi-site grant proposal, and developing improved methods to differentiate between schizophrenia and bipolar disorders.
Traumatic Brain Injury Research at MRN
Mild traumatic brain injury (mTBI) represents a natural union of MRN’s interests in attention, advanced neuroimaging and clinical care. Although there has been much debate about long-term cognitive outcome following mTBI (Dikmen et al., 2009), a recent meta-analysis suggests a moderate effect size (d = .54) for executive, working memory and attention dysfunction during the semi-acute injury phase (Belanger et al., 2005). Laboratory measures suggest that attentional disengagement and selective attention are particularly affected (Drew et al., 2007; Halterman et al., 2006), providing a natural bridge for our in these fields. Second, routine clinical imaging scans (MRI and CT) are usually negative (Belanger et al., 2007; Bigler, 2008; Iverson, 2005), suggesting that these techniques are not sensitive to the pathophysiological changes commonly reported in animal injury models. As a result, alternative neuroimaging techniques are well-positioned to provide unique information about putative “silent lesions” and their impact on cognition functioning.
To this end, published data from MRN provides preliminary evidence of tissue-specific dysfunction and self-reported neuropsychiatric disturbances.This includes increased fractional anisotropy in white matter, likely secondary to cytotoxic edema, secondary inflammatory processes and potential structural alterations in neurofilaments and myelin (Mayer et al., 2010; Ling et al., 2011). White matter creatine and the combined glutamate/glutamine peak (Glx) are also increased (Figure 1), possibly indicative of increased energetics for cell repair (Gasparovic et al., 2009; Yeo et al., 2011). Grey matter is characterized by reduced Glx, potentially representing an adaptive response to avoid excitotoxicity (Gasparovic et al., 2009; Yeo et al., 2011). A tight coupling exists between the release of glutamate into the synaptic cleft, the cycling of glutamate and glutamine between neurons and astrocytes, and the hemodynamic response (Hyder et al., 2006; Mangia et al., 2009; Schummers et al., 2008). Therefore, it is no surprise that mTBI was also associated with hypoactivation of the frontoparietal reorienting network during spatial orienting (Mayer et al., 2009), hypoactivation within the dMFC and lateral PFC during multisensory selective attention (Mayer et al., manuscript in preparation), and behavioral deficits. The balance of intrinsic neuronal fluctuations also appears to be affected by injury (Figure 2), with reduced connectivity in the DMN and increased connectivity in frontoparietal attention networks (Mayer et al., 2011). Given that approximately 80% of the brain’s energy budget is devoted to the cycling of glutamate and glutamine (Hyder et al., 2006; Shulman et al., 2004) and maintaining intrinsic neuronal activity (Raichle and Mintun, 2006; Fox and Raichle, 2007), these functions may be particularly susceptible to diffuse injury following mTBI.
Thus, our current working hypothesis is that a tissue-specific pattern of injury characterizes semi-acute injury, and that these underlying neuronal changes contribute to attentional dysfunction. A key goal of our future work is to determine which changes are most deleterious to cognition. Are orienting and selective attention deficits following mTBI the result of decreased neuronal/metabolic/hemodynamic grey matter response or secondary to a disconnection between frontoparietal sites? Or are cognitive deficits/increased errors following mTBI the result of an imbalance between primary cortical networks mediating internal mentations (DMN) and attention to external events?
Mild TBI also presents an ideal “natural experiment” for examining both the initial consequences of the lesion and the substrate for cognitive recovery (usually 3-6 months post-injury). Results from our lab and others suggest a partial recovery in white matter (Arfanakis et al., 2002; Mayer et al., 2010), with a more robust recovery (Figure 1) of biochemical markers (Vagnozzi et al., 2010; Yeo et al., 2011). We also observed that biochemical recovery is impacted by general cognitive status (Yeo et al., 2011), representing an intriguing possibility about the role of cognitive reserve in mTBI. Finally, reports of persistent hemodynamic abnormalities in clinically asymptomatic mTBI patients (Mayer et al., 2011; McAllister et al., 2006) may explain why a second, temporally proximal mTBI results in greater cognitive deficits than a single injury of comparable severity (Vagnozzi et al., 2008; Wall et al., 2006; Vagnozzi et al., 2005).
Over the past three years, we received 3 NIH awards (R24, R21 and a challenge grant) for our mTBI work. We have amassed a unique multimodal imaging dataset on 55 well-characterized (full neuropsychological and clinical battery) semi-acutely injured mTBI patients and 55 well-matched healthy controls (data collection complete in March 2011), publishing several papers on our initial FMRI, 1H-MRS and DTI findings from our first cohort of patients (samples varied between 16 to 30 patients). The longitudinal aspect of data collection concludes in the fall of 2011, coincidental with the conclusion of our NIH funding. However, much work remains to be done. Replication is acornerstone of science, and our most immediate goal (1-2 years) is to determine the reproducibility of our initial neuroimaging findings in our second independent cohort (25 patients and controls). MEG data was also collected on the second cohort using identical cognitive (evoked) and resting state (intrinsic) tasks, providing an unparalleled dataset for examining the dynamic relationship between electrophysiological and hemodynamic activity. Finally, a basic multisensory task was employed to ensure that widespread hypoactivation during cognitive tasks was not due to differences in basic properties of the hemodynamic response.
A more long-term goal (3-5 years) is to conduct longitudinal imaging studies on the relatively small percentage of patients with “complicated” mTBI. Patients with complicated mTBI have visible focal lesions on CT scans and are more likely to have chronic neuropsychiatric symptoms/poor outcome following injury (Borgaro et al., 2003; Lee et al., 2008; Kashluba et al., 2008; Lange et al., 2009). Current theories (Bigler, 2010; Smits et al., 2008) suggest the focal lesions cause residual cognitive deficits. While intuitive, support for this theory has been mixed (Hughes et al., 2004; Lee et al., 2008; Smits et al., 2008). An alternative theory supported by our work is that poor cognitive outcomes are the result of diffuse injuries in otherwise healthy appearing tissue.