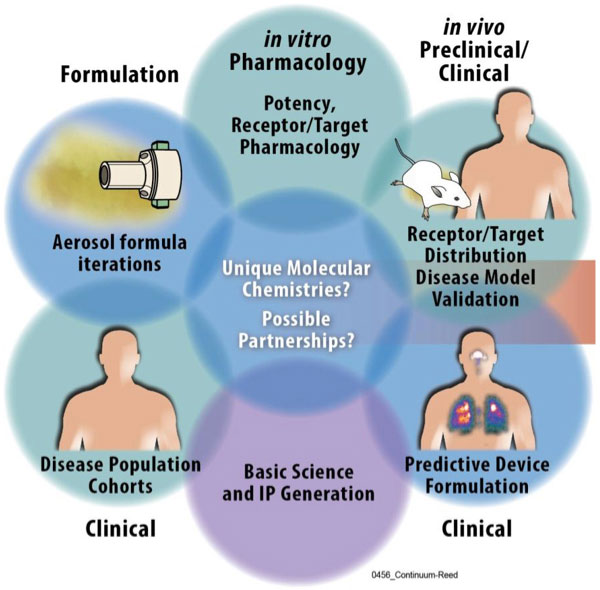
LRRI’s scientists and staff work in a wide range of research disciplines, including immunology, microbiology, aerosol science, chemistry, toxicology; pathology, pathophysiology, physiology; cellular and molecular biology; biostatistics and information sciences. For over 30 years, LRRI has been heavily engaged in the conduct of toxicology studies for government and commercial clients. Early studies conducted by LRRI addressed the health effects of inhaled radionuclides. Later studies addressed the impact of a variety of combustion emission sources, including diesel exhaust and cigarette smoke. LRRI now supports ongoing research for commercial and government customers to explore the inhalation toxicology of pharmaceuticals, chemicals, fuel additives, neurotoxins, and mixtures. LRRI’s scientific emphasis on respiratory health risks concentrates on inhalation toxicology. This includes toxic mechanisms, dosimetry and metabolism, dose response, and interspecies comparisons. LRRI has additional scientific emphasis on inhalation hazard assessment including commercial and defense-related chemicals, product safety testing, EPA compliance, and workplace hazards. LRRI conducts both applied science in respiratory and basic science. Our basic science focuses on causes and cures of disease. We have strengths in pulmonary oncology, chronic obstructive pulmonary disease, lung injury and inflammation.
LRRI’s commercial clients include pharmaceutical companies for whom LRRI scientists perform preclinical Good Laboratory Practice (GLP) studies for submission to the Food and Drug Administration (FDA). Clinical trials are performed nationwide by LRRI scientists at four locations. All trials adhere to FDA specifications.
Lovelace Biomedical and Environmental Research Institute (LBERI) is an award-winning provider of scientific translation research and business solutions for the Federal Government. Our services are organized in the following areas: chemical, biological, and radiological (CBR) research, as well as emerging infectious disease models and studies. These include aerosol laboratory and field studies, Phase I though IV clinical trials in diverse therapeutic areas, and biomedical computer simulation and computing (bioinformatics).